Brain Disorder Diagnosis#
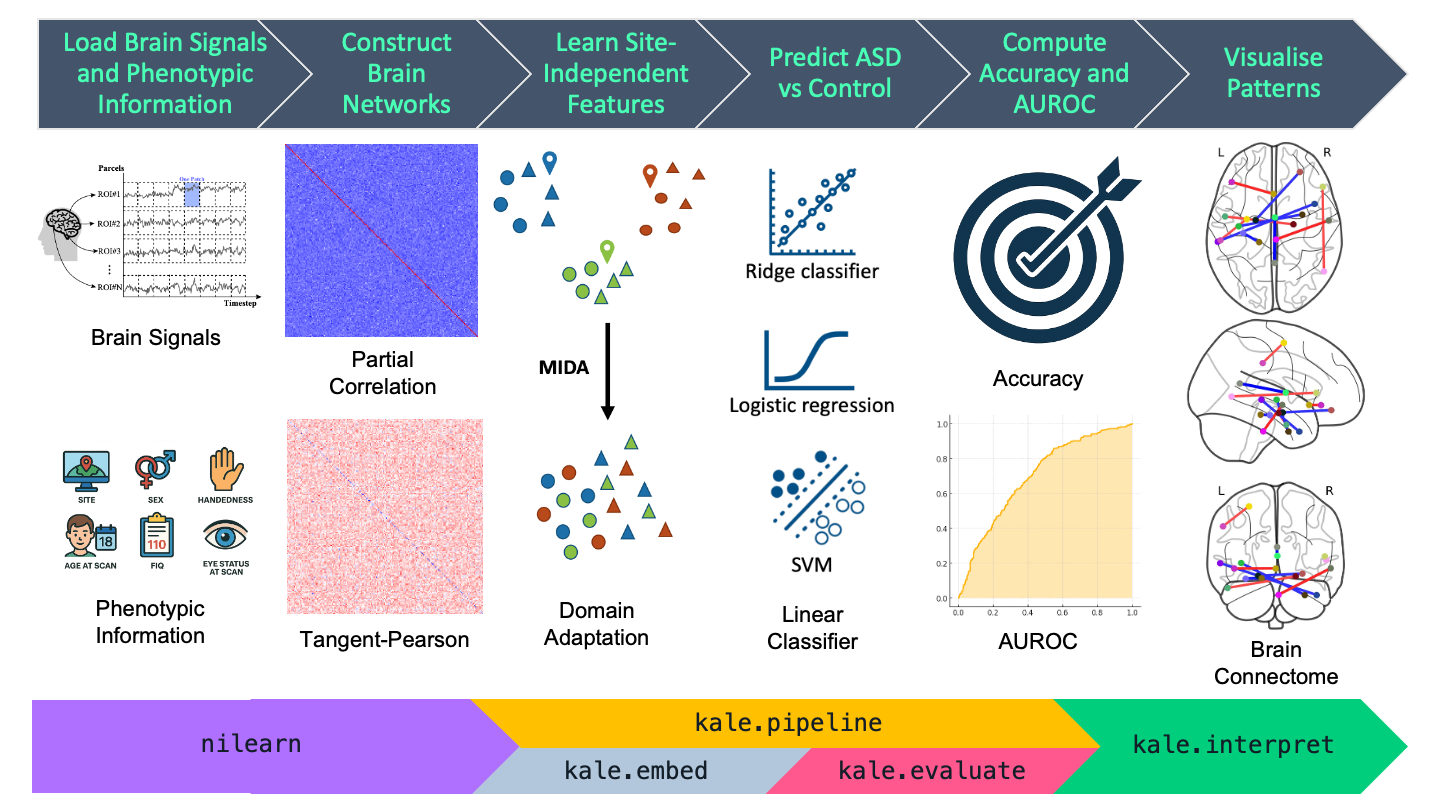
Fig. 1 Machine learning pipeline to predict ASD vs control subjects.#
In this tutorial, we will use PyKale [1] to train models that classify individuals with Autism Spectrum Disorder (ASD) and healthy controls based on two data modalities: functional magnetic resonance imaging (fMRI) and phenotypic features.
We will work with preprocessed data from the Autism Brain Imaging Data Exchange (ABIDE) [2], which includes fMRI and phenotypic information for over 1,000 subjects, contributed by 17 research centers worldwide.
The multimodal approach used in this tutorial involves regularization, where phenotypic features are incorporated into the regularization term to reduce the phenotypic effect (e.g., site effects) in the imaging feature embedding and improve cross-site classification performance.
The main tasks of the tutorial are as follows:
Load fMRI and phenotypic data
Train and evaluate AI models for ASD classification using both data modalities
Explore different cross-validation strategies: random stratified k-fold vs. leave-one-site-out
Apply the method from the TMI paper Kunda et al. (2022) [3], which improves classification performance by reducing data heterogeneity through incorporating phenotypic data into the regularisation of imaging feature embeddings
Visualize the highly weighted features based on model weights for interpretation
Step 0: Environment Preparation#
GPU is not required for this tutorial, you can run the following code cells without changing the runtime type.
To get started, we will install the necessary packages and load a set of helper functions to support the tutorial workflow. Run the cell below to copy all tutorial-related materials to your Google Colab runtime directory. To keep the output clean and focused on interpretation, we will also suppress warnings.
Optional
Learn more about the helper functions used in this tutorial.
Install Required Packages#
Run the cell below to install the required packages for this tutorial. Estimated Runtime in Google Colab: 3-5 minutes
The details for main packages required (excluding PyKale) for this tutorial are:
gdown: A utility package that simplifies downloading files and folders directly from Google Drive.
nilearn: A Python library for neuroimaging analysis. It offers convenient tools for processing, analysing, and visualizing functional MRI (fMRI) data.
yacs: A lightweight configuration management library used to store and organize experiment settings in a hierarchical and human-readable format.
Configuration#
Please refer to the configuration tutorial for general configuration setup instructions for this workshop.
In this tutorial, configs/lpgo/base.yml provides a lightweight setup suitable for quick experimentation. Note that this configuration file is used to describe the outputs shown in this notebook.
Therefore, if a different configuration is used during execution, some of the descriptions or assumptions in the notebook may no longer apply and should be interpreted with caution, as they may become misleading.
Note
The base.yml configuration is designed to yield acceptable performance within 15–25 minutes using a free Google Colab runtime. However, the expected performance will be lower compared to the results reported in Kunda et al. (2022).
For more extensive experimentation, the default hyperparameter grid in config.py provides a broader search space, which may yield improved results at the cost of increased runtime.
If you wish to replicate the exact settings used in Kunda et al. (2022), the configs/lpgo/tmi2022.yml and configs/skf/tmi2022.yml files includes the hyperparameter grid taken directly from their original source code.
A detailed description of each configurable option is provided as optional extended reading in the following sections.
Step 1: Data Loading and Preparation#
Typically, raw fMRI scans require extensive preprocessing before they can be used in a machine learning pipeline. However, the ABIDE dataset provides several preprocessed derivatives, which can be downloaded directly from the Preprocessed Connectomes Project (PCP), eliminating the need for manual preprocessing.
Attention
Given the long runtime required to compute functional connectivity (FC) embeddings from raw fMRI data, this notebook omits that step and instead provides pre-computed embeddings via the load_data function, along with the associated atlas.
For users interested in computing the ROI time series and FC embeddings from scratch, assuming preprocessed images are available, we recommend referring to the following tools and functions:
NiftiLabelsMasker: For use with deterministic (3D) atlases.NiftiMapsMasker: For use with probabilistic (4D) atlases.extract_functional_connectivityfunction inpreprocess.py: This function wrapsnilearn.connectome.ConnectivityMeasureand supports composing multiple FC measures. For example, to compute a Tangent-Pearson embedding from a list of ROI time series, you can call:extract_functional_connectivity(time_series, ["tangent", "pearson"])
Data Loading#
As mentioned earlier, we provide a load_data function to load pre-computed functional connectivity (FC) embeddings along with associated phenotypic information.
This function supports automated downloading via gdown by reading from manifest files if the data is not found locally.
Extended Reading (optional)
Learn more about the configuration arguments for data loading.
from helpers.data import load_data
# A subset of the configuration can be modified here for quick playtest.
# Uncomment the following lines if you are interested in quickly
# modifying the configuration without modifying or making new `yml` files.
# cfg.DATASET.ATLAS = "hcp-ica"
# cfg.DATASET.FC = "tangent-pearson"
# cfg.DATASET.TOP_K_SITES = 5
fc, phenotypes, rois, coords = load_data(
cfg.DATASET.DATA_DIR,
cfg.DATASET.ATLAS,
cfg.DATASET.FC,
top_k_sites=cfg.DATASET.TOP_K_SITES,
)
✔ File found: data/abide/fc/hcp-ica/tangent-pearson.npy
✔ File found: data/abide/phenotypes.csv
✔ Atlas folder found: data/atlas/deterministic/hcp-ica
The downloaded dataset will follow the structure:
dataset_folder/
├── abide/
│ ├── fc/
│ │ └── atlas_name/
│ │ └── fc.npy
│ └── phenotypes.csv
└── atlas/
└── atlas_type/
└── atlas_name/
├── atlas.nii.gz
├── coords.npy
└── labels.txt
Descriptions for each file are as follows:
abide/fc/atlas_name/fc.npy
A NumPy array containing functional connectivity (FC) matrices for all subjects using a specific atlas. Each FC is typically a symmetric matrix of shape(regions × regions).abide/phenotypes.csv
A CSV file with subject-level metadata, including attributes such asage,sex,diagnosis group, andsite. This information is essential for downstream analyses like classification or covariate correction.atlas/atlas_type/atlas_name/atlas.nii.gz
A NIfTI file representing the brain parcellation. Each voxel value corresponds to a labeled brain region defined by the atlas.atlas/atlas_type/atlas_name/coords.npy
A NumPy array with shape(N, 3)representing the MNI coordinates of region centroids. These are often used in graph construction or spatial visualization.atlas/atlas_type/atlas_name/labels.txt
A plain text file listing the names of the brain regions defined in the atlas, with one region name per line.
While we use .npy files for fc and coords, our pipeline does support different file formats like .csv, as long as the loaded data conforms to the expected format required by the corresponding functions or methods.
Exercise 1
How many samples are found in the sub-sampled ABIDE dataset?
Hint
In Python, the length of arrays like lists and tuples can be found using
len(array).The
phenotypesvariable is an array containing the phenotypes describing the subjects.
To get a more visual overview on what FC represents and which parts of it we use for the features, we visualize the FC below using a heatmap.
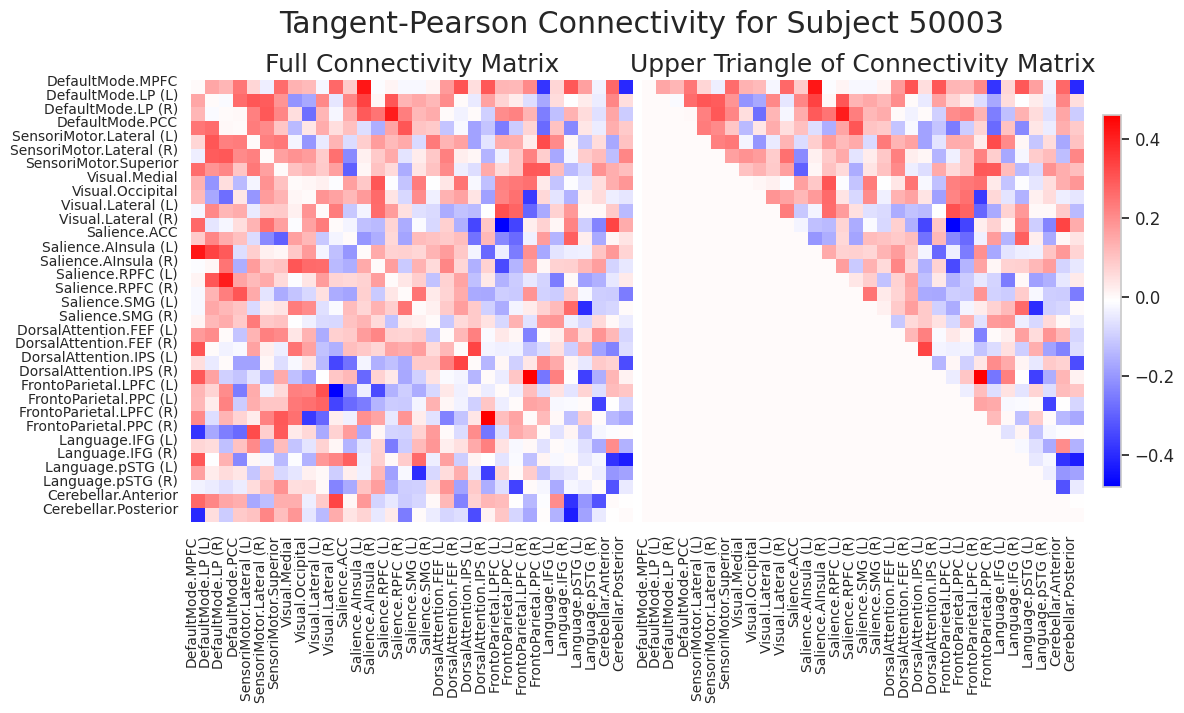
The heatmap above displays the functional connectivity (FC) matrix for Subject 50003, computed using the Tangent-Pearson method. The matrix represents pairwise relationships between different brain regions of interest (ROIs) based on their time-series similarity.
Left panel – Full Connectivity Matrix:
A symmetric matrix where each entry represents the strength and direction of FC between two ROIs.Red values indicate positive connectivity.
Blue values indicate negative connectivity.
The matrix is symmetric because the connectivity from region A to B is equal to that from B to A.
Right panel – Upper Triangle of the Matrix:
To avoid redundancy due to symmetry, only the upper triangular portion of the matrix (excluding the diagonal) is shown.
This representation is commonly used in machine learning pipelines to vectorize the FC matrix for classification or regression tasks, significantly reducing the number of features fromn*nton*(n-1)/2, wherenis the number of ROIs. However, the feature size increase will remainO(n^2)as the number of ROIs increases.Colorbar:
Indicates the range of connectivity values, with stronger connections lying at the extremes of red and blue.
This representation is widely used in neuroimaging studies for subject-level modeling, feature extraction, and biomarker discovery.
Exercise 2
How many ROIs are defined in the FC matrix?
Hint
In Python, the length of arrays like lists and tuples can be found using
len(array).The
roisvariable is an array containing the label for each available ROI.
Next, we want to inspect the phenotypic information provided in ABIDE dataset.
| SUB_ID | X | subject | SITE_ID | FILE_ID | DX_GROUP | DSM_IV_TR | AGE_AT_SCAN | SEX | HANDEDNESS_CATEGORY | ... | qc_notes_rater_1 | qc_anat_rater_2 | qc_anat_notes_rater_2 | qc_func_rater_2 | qc_func_notes_rater_2 | qc_anat_rater_3 | qc_anat_notes_rater_3 | qc_func_rater_3 | qc_func_notes_rater_3 | SUB_IN_SMP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 218 | 50302 | 226 | 50302 | UM_1 | UM_1_0050302 | 1 | -9999 | 10.4000 | 2 | R | ... | NaN | OK | NaN | OK | NaN | OK | NaN | OK | NaN | 0 |
| 373 | 50501 | 410 | 50501 | USM | USM_0050501 | 1 | 1 | 17.7057 | 1 | NaN | ... | NaN | OK | NaN | OK | NaN | OK | NaN | OK | NaN | 1 |
| 594 | 50954 | 646 | 50954 | NYU | NYU_0050954 | 1 | 1 | 14.7500 | 2 | NaN | ... | NaN | OK | NaN | OK | NaN | OK | NaN | OK | NaN | 0 |
| 931 | 51333 | 1003 | 51333 | MAX_MUN | MaxMun_c_0051333 | 2 | 0 | 24.0000 | 1 | R | ... | NaN | OK | NaN | maybe | ic-cerebellum | OK | NaN | OK | NaN | 1 |
| 14 | 50017 | 16 | 50017 | PITT | Pitt_0050017 | 1 | 1 | 22.7000 | 1 | R | ... | NaN | maybe | skull-striping fail | maybe | ic-cerebellum_temporal_lob | fail | noise | OK | NaN | 1 |
5 rows × 104 columns
As we can see, there is a wide range of phenotypic information available for each subject ranging from patient descriptors such as site (SITE_ID), diagnostic group (DX_GROUP), and age at scan (AGE_AT_SCAN), to quality control metrics for individual scans (e.g., columns starting with qc).
Exercise 3
How many phenotypic variables are available in the ABIDE dataset?
We also want to know how the phenotypes are distributed, we can visualize it with count and histogram plot for categorical and continuous variable respectively. Following Kunda et al. (2022), we mainly focused on the distribution of site, gender, handedness, eye status, age, and FIQ.
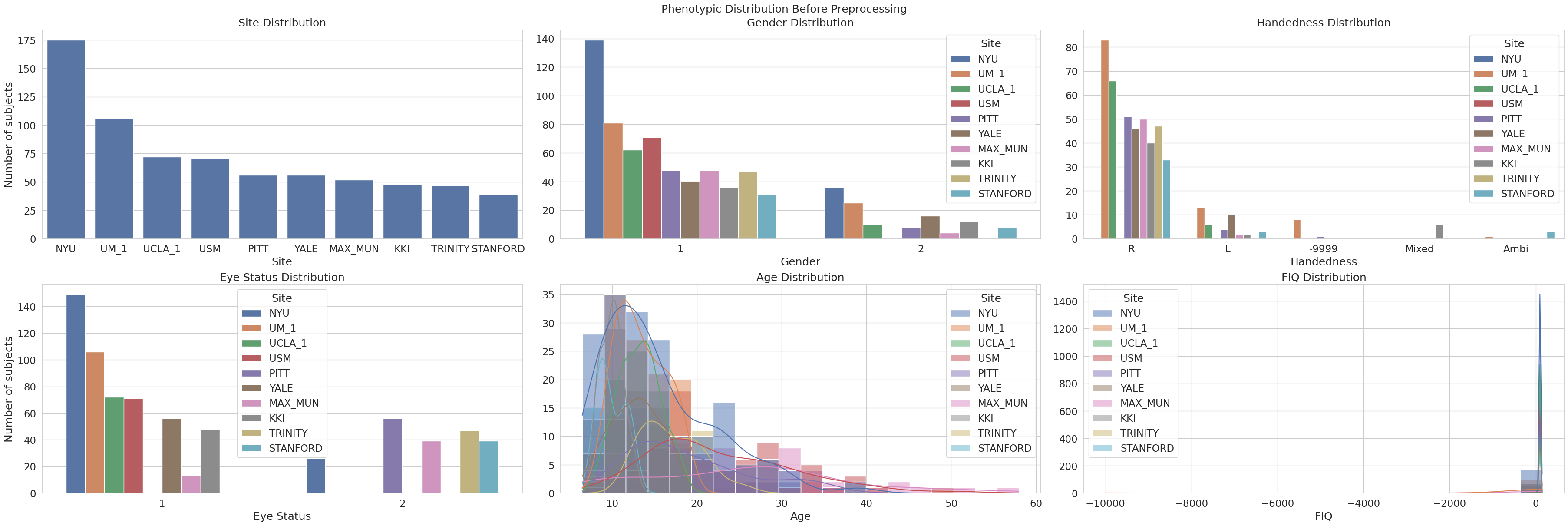
Several important observations can be made:
Site Distribution: The majority of subjects were collected at NYU, followed by UM_1 and UCLA_1. Other sites like USM, PITT, and YALE have relatively fewer samples. This imbalance in sample size could bias model performance toward larger sites if not properly addressed through harmonization or site-stratified validation.
Gender Distribution: Across nearly all sites, the dataset is male-dominated (
1= male,2= female), a known characteristic of ABIDE. The underrepresentation of females could limit the generalizability of sex-related findings.Handedness Distribution: Most subjects are right-handed (
R), with smaller proportions of left-handed (L), ambidextrous (Ambi), and mixed. Notably, there is a substantial number of-9999entries, indicating missing or invalid data. This missingness is uneven across sites, potentially introducing site-specific biases.Eye Status Distribution: Most scans were recorded with subjects’ eyes open (
1), though a non-negligible number had eyes closed (2). The distribution is generally consistent across sites, with minimal missing data.Age Distribution: The age of participants ranges from around 5 to 55 years, with a strong skew toward younger subjects, especially between 7 and 18 years old. This is typical of developmental neuroimaging datasets and emphasizes the need to control for age in modeling or analysis.
FIQ (Full-Scale IQ) Distribution: The FIQ distribution is severely distorted by missing or placeholder values (e.g.,
-9999). These dominate the histogram and create an artificial spike around zero. Valid FIQ values span a wide range but are sparsely distributed. Imputation or exclusion of these invalid entries is essential for any analysis involving IQ.
Data Preprocessing#
Before modeling, we need to preprocess the phenotypic variables to ensure they are in a usable format. This includes handling missing values, encoding categorical variables, and optionally standardizing continuous ones.
Optional
Learn more about the categorical variables from phenotypic data used in this tutorial.
from helpers.preprocess import preprocess_phenotypic_data
# A subset of the configuration can be modified here for quick playtest.
# Uncomment the following lines if you are interested in quickly
# modifying the configuration without modifying or making new `yml` files.
# cfg.PHENOTYPE.STANDARDIZE = "site"
labels, sites, phenotypes = preprocess_phenotypic_data(
phenotypes, cfg.PHENOTYPE.STANDARDIZE
)
After preprocessing, we want to observe how the encoding, imputation, and standardization affected the phenotypes.
| AGE_AT_SCAN | FIQ | SITE_ID_KKI | SITE_ID_MAX_MUN | SITE_ID_NYU | SITE_ID_PITT | SITE_ID_STANFORD | SITE_ID_TRINITY | SITE_ID_UCLA_1 | SITE_ID_UM_1 | SITE_ID_USM | SITE_ID_YALE | SEX_FEMALE | SEX_MALE | HANDEDNESS_CATEGORY_AMBIDEXTROUS | HANDEDNESS_CATEGORY_LEFT | HANDEDNESS_CATEGORY_RIGHT | EYE_STATUS_AT_SCAN_CLOSED | EYE_STATUS_AT_SCAN_OPEN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUB_ID | |||||||||||||||||||
| 50302 | -1.046379 | -0.566951 | False | False | False | False | False | False | False | True | False | False | True | False | False | False | True | False | True |
| 50501 | -0.602117 | -1.553855 | False | False | False | False | False | False | False | False | True | False | False | True | False | False | True | False | True |
| 50954 | -0.078069 | -2.174853 | False | False | True | False | False | False | False | False | False | False | True | False | False | False | True | False | True |
| 51333 | -0.111109 | -0.991772 | False | True | False | False | False | False | False | False | False | False | False | True | False | False | True | True | False |
| 50017 | 0.546855 | -1.911015 | False | False | False | True | False | False | False | False | False | False | False | True | False | False | True | True | False |
Now we can see that the number of phenotypes are now reduced, the continuous and categorical variables are now standardized and one-hot encoded respectively.
Exercise 4
How many phenotypes are there once we have preprocessed the phenotypes?
Hint
In
pandas, executingpd.DataFrame.shapeoutputs atuplecontaining(num_rows, num_columns).The
phenotypesvariable is apd.DataFrametype.
Exercise 5
We have seen the preprocessed phenotypes and noted that the categorical variables have been one-hot-encoded.
Given your observation, what does one-hot encoding do to the categorical variables?
We also want to check how the phenotypes are distributed after we preprocess it.
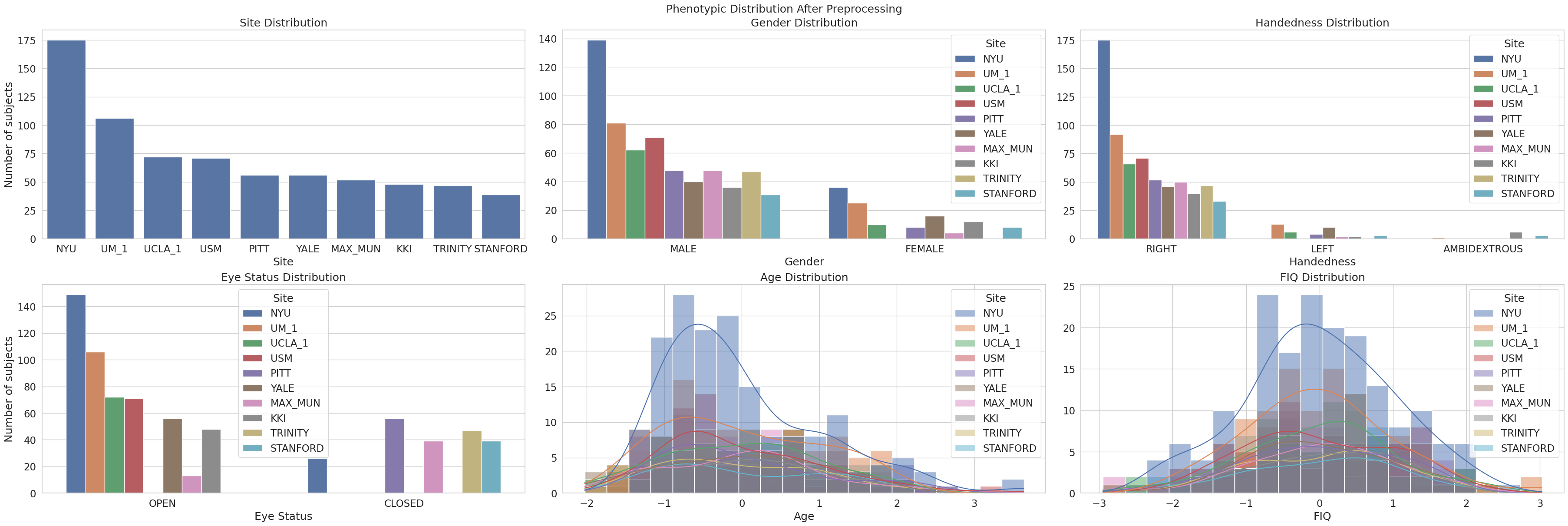
We can see that we can interpret the phenotypes much clearer now, as we can infer that:
Site Distribution: The overall site imbalance remains, with NYU contributing the largest number of subjects, followed by UM_1 and UCLA_1. This is expected given no missing values for the site label.
Gender Distribution: The gender imbalance persists post-preprocessing, with male subjects still forming the majority at each site. Like site, its distribution remains the same as there are no missing values.
Handedness Distribution: The preprocessing step appears to have removed most invalid or missing values, particularly entries like
-9999. The dataset now primarily includes right-handed subjects, with a small proportion of left-handed and ambidextrous individuals. This results in a cleaner handedness distribution, thus also reducing the dimension size of one-hot encoded handedness.Eye Status Distribution: The eye status remains consistent, with most subjects scanned with eyes open. Very few entries are labeled with eyes closed, and no missing values are apparent, suggesting good data completeness for this variable.
Age Distribution: Age values have been normalized, and the distribution now appears centered around zero (z-scored). The skew toward younger participants is still present, but more subtle. This normalization facilitates fair comparison across sites and removes scale bias in modeling.
FIQ (Full-Scale IQ) Distribution: Similar to age, FIQ has been standardized, producing a roughly normal distribution across sites. The spike of invalid values (
-9999) observed in the raw data has been eliminated, indicating effective handling of missing or outlier values during preprocessing. This cleaner distribution is more suitable for downstream statistical analysis and machine learning models.
Step 2: Model Definition#
PyKale provides flexible pipelines for modelling interdisciplinary problems. In our case, the primary objective is to develop a robust yet interpretable model capable of effectively integrating multi-site data.
We leverage the kale.pipeline.multi_domain_adapter.AutoMIDAClassificationTrainer (or simply Trainer), which encapsulates the domain adaptation method Maximum Independence Domain Adaptation (MIDA) [4]. This trainer integrates domain adaptation with classification by allowing the user to specify any scikit-learn-compatible linear classifier for prediction, offering a convenient way to construct powerful and flexible pipelines.
In the following sections, we first describe the cross-validation split strategy used in this tutorial, followed by an explanation of the embedding extraction and prediction methods.
Cross-Validation Split#
The choice of cross-validation strategy can significantly impact how can we evaluate of a model’s robustness and generalizability, especially when dealing with multi-site or grouped data.
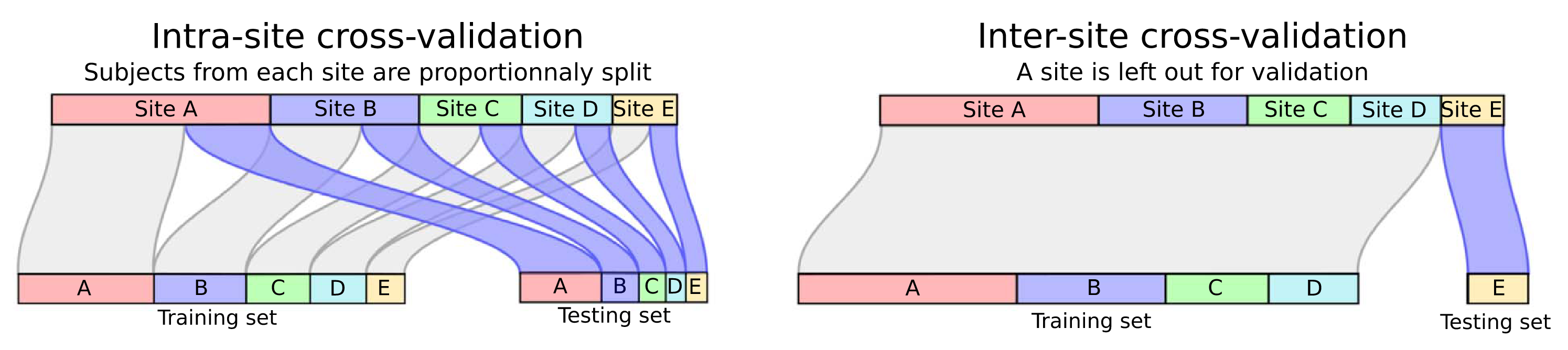
Fig. 2 Illustrative comparison between random k-fold and leave-one-site-out [5].#
The figure above compares two different cross-validation strategies which we will consider for this tutorial:
n-Repeated Stratified k-Fold (SKF):
This method ensures that each fold maintains the original label distribution (e.g., equal proportion of+and−classes). However, it does not guarantee that data from the same group (e.g., site, subject, scanner) are kept together, potentially leading to data leakage if the same group appears in both train and test splits.Leave-One-Site-Out or Leave p-Groups Out (LPGO): This method preserves the group structure by leaving out entire groups during each iteration. It is particularly suited for evaluating generalization to unseen sites or subjects, as it avoids group leakage. However, it may result in imbalanced label distributions in each fold.
Each method serves a different purpose: stratified k-fold is ideal when label balance is critical, while leave-p-groups-out is better for assessing model robustness under domain shift or site variability. Realistically, LPGO is preferable given real data will most likely not have the same distribution as a model’s training data.
Exercise 6
Consider we evaluate a model using SKF with two repetition and five folds or LPGO with ten groups with one group left out for testing, we will need to train a total of ten models. If we evaluate a model using:
SKF with five repetition and ten folds
LPGO with five groups and two groups left out for testing
How many models we have to train for each cases?
Hint
For LPGO, given m total groups and p left out groups, consider it as a combinatorial problem.
Optional
Learn more about the configuration arguments for cross-validation used in this tutorial.
from sklearn.model_selection import LeavePGroupsOut, RepeatedStratifiedKFold
# A subset of the configuration can be modified here for quick playtest.
# Uncomment the following lines if you are interested in quickly
# modifying the configuration without modifying or making new `yml` files.
# cfg.CROSS_VALIDATION.SPLIT = "skf"
# cfg.CROSS_VALIDATION.NUM_FOLDS = 5
# cfg.CROSS_VALIDATION.NUM_REPEATS = 2
# Define the default cross-validation strategy:
# Repeated stratified k-fold maintains class distribution across folds and supports multiple repetitions
cv = RepeatedStratifiedKFold(
# Number of stratified folds
n_splits=cfg.CROSS_VALIDATION.NUM_FOLDS,
# Number of repeat rounds
n_repeats=cfg.CROSS_VALIDATION.NUM_REPEATS,
# Ensures reproducibility, intentionally set to the seed to have the same splits across runs
random_state=cfg.RANDOM_STATE,
)
# Override with leave-p-proups-out if specified
# This strategy holds out `p` unique groups (e.g., sites) per fold, enabling group-level generalization
if cfg.CROSS_VALIDATION.SPLIT == "lpgo":
# Use group-based CV for domain adaptation or site bias evaluation
cv = LeavePGroupsOut(cfg.CROSS_VALIDATION.NUM_FOLDS)
Embedding Extraction#
Domain adaptation aims to reduce distributional discrepancies between datasets collected under different conditions (e.g., sites, scanners, protocols). This helps ensure that the learned representations generalize across domains.
MIDA was originally proposed by Yan et al. (2017) [4] in IEEE Transactions on Cybernetics to reduce time-varying drift in sensors, using domain features such as device label and acquisition time.
Kunda et al. (2022) extended MIDA for neuroimaging studies, enabling multi-domain adaptation for multi-site data integration.
PyKale includes a scikit-learn-style implementation of MIDA in kale.embed.factorization.MIDA, adopting a similar interface to KernelPCA to ensure interoperability, extensive customization, and ease of use.
Prediction Methods#
To maintain compatibility and user-friendliness, PyKale supports linear classifiers from scikit-learn, including:
Logistic Regression (LR)
Support Vector Machines (SVM)
Ridge Classifier (Ridge)
These models can be selected easily by passing the appropriate string identifier, streamlining experimentation with different classifiers.
Linear classifiers are particularly suitable in this context due to their inherent interpretability. Its coefficients can be directly inspected to understand the contribution of each feature to the prediction.
Baseline and Proposed Model#
We define several model configurations used for classification. Each model shares the same base classifier but differs in how domain adaptation is applied:
Baseline: A standard model trained directly on functional connectivity features without domain adaptation.
Site Only: A domain-adapted model that uses site labels as the adaptation factor to reduce site-specific bias.
All Phenotypes: An extended domain-adapted model that incorporates multiple phenotypic variables (e.g., age, sex, handedness) to further reduce inter-site variability.
Optional
Learn more about the hyperparameter grid used in this tutorial.
from sklearn.base import clone
from kale.pipeline.multi_domain_adapter import AutoMIDAClassificationTrainer as Trainer
from helpers.parsing import parse_param_grid
# A subset of the configuration can be modified here for quick playtest.
# Uncomment the following lines if you are interested in quickly
# modifying the configuration without modifying or making new `yml` files.
# cfg.MODEL.CLASSIFIER = "lr"
# cfg.MODEL.PARAM_GRID = None
# cfg.MODEL.NUM_SEARCH_ITER = 100
# cfg.MODEL.NUM_SOLVER_ITER = 100
# Configuration with cv and random_state/seed included
trainer_cfg = {k.lower(): v for k, v in cfg.TRAINER.items() if k != "PARAM_GRID"}
trainer_cfg = {**trainer_cfg, "cv": cv, "random_state": cfg.RANDOM_STATE}
# Initialize dictionary for different trainers
trainers = {}
# Create a baseline trainer without domain adaptation (MIDA disabled)
param_grid = parse_param_grid(cfg.TRAINER.PARAM_GRID, "domain_adapter")
trainers["baseline"] = Trainer(use_mida=False, param_grid=param_grid, **trainer_cfg)
# Create a trainer with MIDA enabled, using site labels as domain adaptation factors
param_grid = parse_param_grid(cfg.TRAINER.PARAM_GRID)
trainers["site_only"] = Trainer(use_mida=True, **trainer_cfg)
# Clone the 'site_only' trainer to create 'all_phenotypes' trainer
# This enables reusing the same training configuration, while modifying only the input domain factors
trainers["all_phenotypes"] = clone(trainers["site_only"])
Step 3: Model Training#
The Trainer automatically handles model training and hyperparameter tuning based on the specified cross-validation strategy. To initiate training, simply call the fit(...) method, which accepts the following arguments:
x: The input features used for training and tuning the model.y: The target labels corresponding to each sample.groups: Group identifiers for each sample, used specifically in group-aware cross-validation methods such as Leave-p-Groups-Out (LPGO).group_labels: Additional metadata or domain features describing each sample (e.g., phenotypes, one-hot encoded site indicators) used by the domain adaptation method.
This interface allows seamless integration of domain information and supports robust validation across multi-site datasets.
As noted earlier, we evaluate three model variants:
For the baseline model, no additional
group_labelsare required, as domain adaptation is not applied.The site only and all phenotypes models do require
group_labelsto be specified in order to enable domain adaptation using site or phenotypic information.
Given that we have already preprocessed the phenotypic data and extracted site labels, we can pass the appropriate group_labels during training:
Use
sitesfor the site only model.Use the full
phenotypesdata (including one-hot encoded site and demographic features) for the all phenotypes model.
This demonstrates that the Trainer provides flexible control over the use of MIDA, allowing users to choose whether or not to incorporate domain adaptation based on the available metadata and the specific goals of their analysis.
Estimated Runtime in Google Colab: 18-25 minutes
import pandas as pd
from tqdm import tqdm
# Define common training arguments for all models: features (X), labels (y), and group info (sites)
fit_args = {"x": fc, "y": labels, "groups": sites}
cv_results = {}
for model in (pbar := tqdm(trainers)):
args = clone(fit_args, safe=False)
if model == "site_only":
args["group_labels"] = sites
if model == "all_phenotypes":
args["group_labels"] = phenotypes
pbar.set_description(f"Fitting {model} model")
trainers[model].fit(**args)
cv_results[model] = pd.DataFrame(trainers[model].cv_results_)
Fitting all_phenotypes model: 100%|██████████| 3/3 [00:38<00:00, 13.00s/it]
Once the models are simultaneously trained and tuned, the cross-validation results are stored in the cv_results_ attribute. These results are automatically sorted according to the metric specified in the refit argument.
The cv_results_ object contains comprehensive information, including:
The hyperparameter configurations explored during tuning.
Performance scores for each split.
Aggregated statistics such as the mean and standard deviation across folds.
This allows for detailed inspection and comparison of model performance across different hyperparameter settings.
If we are only interested in a single evaluation metric, we can use the best_score_ attribute to retrieve the best average performance across all splits based on that metric.
To facilitate comparison across models, we aggregate each model’s cv_results_ into a dict of pd.DataFrame objects. These can then be compiled into a summary table that highlights the best-tuned performance for each of the three evaluated models.
Exercise 7
Can you mention what are the available aggregates for each metrics found in cv_results_?
Hint
You can inspect cv_results_ or cv_results[model] just like phenotypes.
Step 4: Evaluation#
After training and tuning the models, we evaluate the performance of the three model configurations using accuracy as the primary metric for comparison.
We compile the top-performing scores from cross-validation for each model, allowing us to assess the effectiveness of different domain adaptation strategies. By comparing models with and without domain adaptation, we can examine the impact of incorporating site and phenotypic information on multi-site autism classification performance.
This can be done using the compile_results function, which summarizes cross-validation outputs into a clean and comparable format. The function accepts the following:
cv_results: A dictionary that maps model names (e.g.,"Baseline","Site Only","All Phenotypes") to their cross-validation results. These can either bepandas.DataFrameobjects or nested dictionaries with performance scores.sort_by: The metric used to select the best-performing configuration for each model. Supported metrics include"accuracy","precision","recall","f1","roc_auc", and"matthews_corrcoef".
It returns a pd.DataFrame where each row corresponds to a model, and each column shows the formatted score (e.g., mean ± std) for the selected metric.
This analysis highlights which configurations generalize best across heterogeneous imaging sites.
In addition, we report performance from an experiment using 2-repeated stratified 5-fold cross-validation, which can be run by loading the configuration file at experiments/skf/base.yml. As expected, the performance differences are less pronounced in this setting. This is likely because blending data from different sites maintains label distribution but does not reflect a realistic evaluation of generalization under domain shift, a scenario encountered when deploying models to unseen sites.
Model |
Accuracy |
AUROC |
|---|---|---|
Baseline |
0.6711 ± 0.0330 |
0.7295 ± 0.0238 |
Site Only |
0.6877 ± 0.0357 |
0.7372 ± 0.0228 |
All Phenotypes |
0.6849 ± 0.0314 |
0.7396 ± 0.0215 |
The key question now is: Does domain adaptation actually improve predictive performance under a leave-one-group-out setting, where generalization to unseen sites is critical?
from helpers.parsing import compile_results
# Compile the cross-validation results into a summary table,
# sorting by the model with the highest test accuracy across CV folds
compiled_results = compile_results(cv_results, "accuracy")
# Display the compiled results DataFrame (models as rows, metrics as formatted strings)
display(compiled_results)
| Accuracy | AUROC | |
|---|---|---|
| Model | ||
| Baseline | 0.6678 ± 0.0982 | 0.7152 ± 0.0883 |
| Site Only | 0.6960 ± 0.0884 | 0.7233 ± 0.0925 |
| All Phenotypes | 0.6902 ± 0.0948 | 0.7241 ± 0.0884 |
Turns out, domain adaptation indeed helps when evaluated under the leave-one-group-out setting.
We observe a consistent performance improvement when incorporating site and phenotypic information:
The Site Only model achieves the highest accuracy, indicating that accounting for site differences is beneficial for generalization.
The All Phenotypes model also outperforms the Baseline, suggesting that additional phenotypic features contribute useful domain information, although the performance difference over site alone is marginal given the accuracy and AUROC.
The Baseline model, which does not use domain adaptation, performs worst, highlighting the challenge of multi-site variability when no adaptation is applied.
These results demonstrate the effectiveness of domain adaptation in improving model generalization across imaging sites, especially in scenarios where each site may exhibit different data distributions.
Step 5: Interpretation#
We interpret the trained models by analyzing the learned weights associated with functional connectivity features. Specifically, we extract the top-weighted ROI pairs that contribute most to the classification decision.
These weights are visualized using a connectome plot, which helps reveal the brain region interactions that are most informative for distinguishing individuals with autism from neurotypical controls. This enhances the interpretability of the model and may offer insights into neurobiological patterns relevant to autism.
To support this, PyKale extends nilearn’s plot_connectome through the utility kale.interpret.visualize.visualize_connectome. This enhanced version improves interpretability by:
Adding annotations for each ROI.
Visualizing only the top-weighted connections between regions, making the plot more focused and informative.
This visualization aids both in model transparency and in deriving neuroscientific interpretations from machine learning outputs.
import numpy as np
from kale.interpret.visualize import visualize_connectome
# Fetch model with best performance
best_model = max(cv_results, key=lambda m: trainers[m].best_score_)
# Fetch coefficients to visualize feature importance
coef = trainers[best_model].coef_.ravel()
# check if coef != features, assumes augmented features with phenotypes/sites
if coef.shape[0] != fc.shape[1]:
coef, _ = np.split(coef, [fc.shape[1]])
# Visualize the coefficients as a connectome plot
proj = visualize_connectome(
coef,
rois,
coords,
1.5 / 100, # Take top 1.5% of connections
legend_params={
"bbox_to_anchor": (3.5, -0.4), # Align legend outside the plot
"ncol": 2,
},
)
# Display the resulting connectome plot
display(proj)
<nilearn.plotting.displays._projectors.OrthoProjector at 0x71a6c04c3d00>
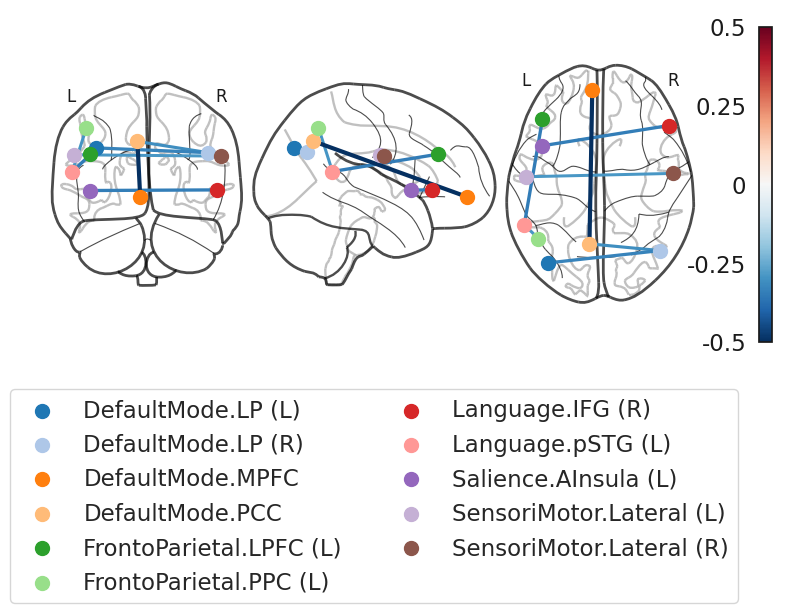
The figure illustrates the most discriminative ROI-to-ROI functional connections that differentiate Autism Spectrum Disorder (ASD) participants from Controls, based on group-level functional connectivity analysis.
Blue edges indicate stronger functional connectivity in Control participants.
Red edges (not present in this figure) would indicate stronger connectivity in ASD.
The color saturation and thickness of the edges represent the magnitude of discriminative contribution of each connection.
Key Observations
Default Mode Network (DMN)
Includes: DefaultMode.MPFC, DefaultMode.PCC, DefaultMode.LP (L/R)
Several intra-DMN and interhemispheric connections appear weaker in ASD, aligning with known disruptions in self-referential thinking and social cognition.
Fronto-Parietal Network
Includes: FrontoParietal.LPFC (L), FrontoParietal.PPC (L)
Weaker connectivity in this network may indicate impaired executive function and cognitive control, both commonly reported in ASD.
Language Network
Includes: Language.IFG (R), Language.pSTG (L)
Reduced connections involving language-related areas suggest communication challenges in ASD.
Salience and Sensorimotor Networks
Includes: Salience.AInsula (L), SensoriMotor.Lateral (L/R)
Altered connectivity in these regions may reflect atypical sensory integration and interoception, frequently observed in autistic individuals.
Interpretation Considerations
While these patterns provide evidence for disrupted large-scale network integration in ASD, caution is warranted:
Regions such as sensorimotor cortex are known to be susceptible to head motion and site-related variability.
The presence of domain adaptation and harmonization in the modeling pipeline reduces, but does not fully eliminate these confounds.
Therefore, while findings involving DMN and language networks are likely robust, sensorimotor findings should be interpreted with care.
Summary
This figure underscores a consistent pattern of reduced functional connectivity across the Default Mode, Language, Fronto-Parietal, and Sensorimotor networks in ASD. These disruptions support the theory of altered network-level integration in autism and bolster the potential of connectivity-based biomarkers for diagnostic classification.
References#
[1] Lu, H., Liu, X., Zhou, S., Turner, R., Bai, P., Koot, R. E., … & Xu, H. (2022, October). PyKale: Knowledge-aware machine learning from multiple sources in Python. In Proceedings of the 31st ACM International Conference on Information & Knowledge Management (pp. 4274-4278).
[2] Craddock, C., Benhajali, Y., Chu, C., Chouinard, F., Evans, A., Jakab, A., … & Bellec, P. (2013). The neuro bureau preprocessing initiative: open sharing of preprocessed neuroimaging data and derivatives. Frontiers in Neuroinformatics, 7(27), 5.
[3] Kunda, M., Zhou, S., Gong, G., & Lu, H. (2022). Improving multi-site autism classification via site-dependence minimization and second-order functional connectivity. IEEE Transactions on Medical Imaging, 42(1), 55-65.
[4] Yan, K., Kou, L., & Zhang, D. (2017). Learning domain-invariant subspace using domain features and independence maximization. IEEE transactions on cybernetics, 48(1), 288-299.
[5] Abraham, A., Milham, M. P., Di Martino, A., Craddock, R. C., Samaras, D., Thirion, B., & Varoquaux, G. (2017). Deriving reproducible biomarkers from multi-site resting-state data: An Autism-based example. NeuroImage, 147, 736-745.

